Our Research
Our lab designs and develops DNA-inspired Janus base nanomaterials (JBNs) for a variety of biomedical applications.
1) Janus Base Nanotube (JBNt): molecular engineering using medicinal chemistry and computational modeling approaches.
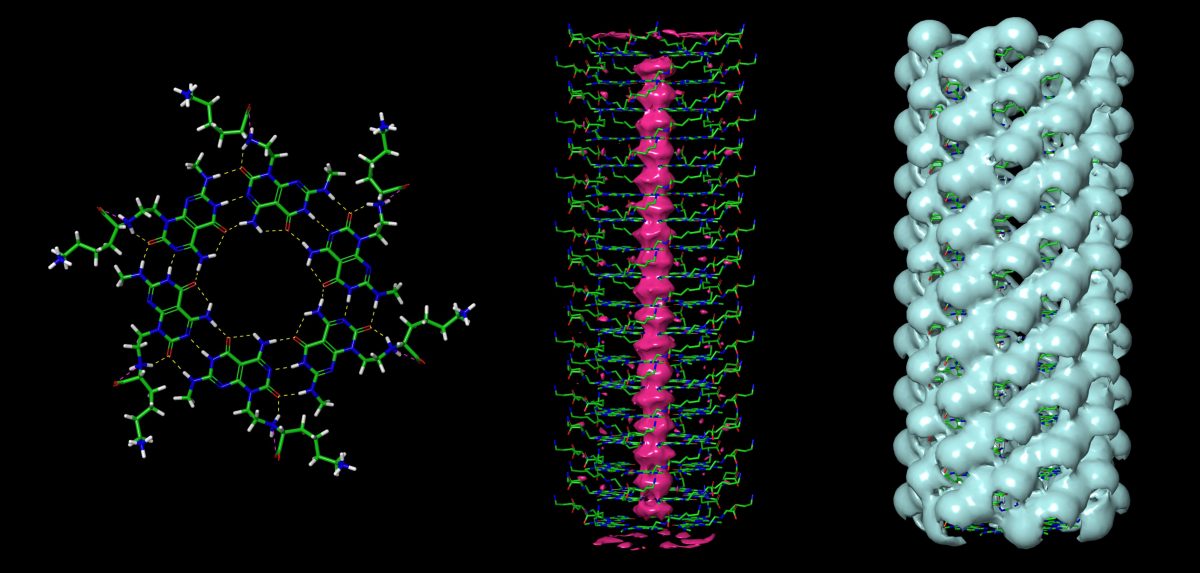
Utilizing molecular design, organic synthesis and computational modeling approaches, we design, synthesize and characterize a family of small molecules with multiple hydrogen donor-acceptor pairs, named “Janus bases”. Mimicking DNA base pairs, these Janus bases can self-assemble into various 2D nanorosettes and 3D nanotubes (as shown below).
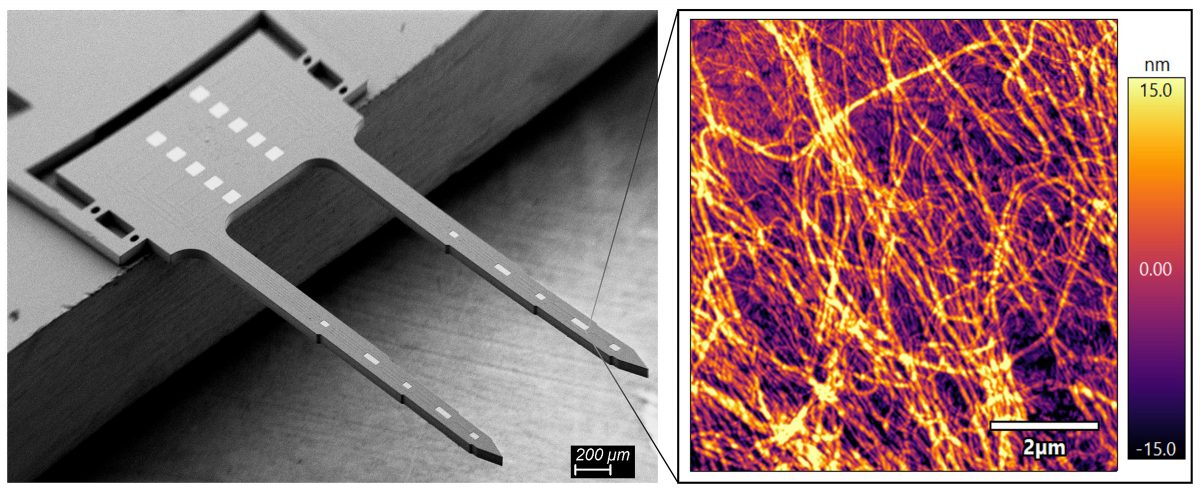
2) Janus Base Nano-coating (JBNc): electrically conductive and pizeoelectric coating for improved electrode surface and neurological applications.
For the first time, we developed an electrical conductive coating based on biomolecules—JBNc. The electrochemical properties of JBNc met or exceeded the standards of conventional gold and iridium oxide electrode surface. Moreover, JBNc can dramatically reduce the stiffness of the electrode surface to an extend close to the natural brain tissue. Especially, JBNc is a bioactive coating that can bioactivity promote neuron adhesion, neurogenesis, ion channel and anti-apoptosis.
3) Janus Base Nanoparticle (JBNp): A versatile delivery platform for CRISPR and RNA therapeutics
Because JBNps are formed by non-covalent interactions between small molecule Janus Base units, they present significantly lower cytotoxicity compared to conventional polymers, carbon nanotubes (CNTs) and lipid nanoparticles (LNPs). More importantly, JBNps can escape from early endosomes via a pore formation mechanism. This is the molecular basis for why JBNp can be more effective in delivering large mRNA cargos (such as Cas9 mRNA) than conventional lipid nanoparticles. With this capability, JBNp offer a practical, non-viral way to bring gene editing tools (CRISPR) into cells: they can carry the genetic instructions for Cas9 mRNA together with a guide RNA (sgRNA) that directs the editor to a specific, harmful DNA sequence, enabling targeted correction at its source. We are currently evaluating this approach in proof-of-concept studies focused on liver and kidney gene editing, where safe and efficient delivery remains one of the key barriers to translating gene editing into real-world therapies.
Most drug-delivery nanoparticles are spherical, which can limit their ability to infiltrate hard-to-penetrate tissues such as solid tumors or cartilage with a dense extracellular matrix (ECM). To overcome this barrier, we develop rod-shaped JBNp formulations to improve tissue penetration and targeting to treat solid ovarian tumors. In parallel, rod-shaped JBNps are being developed to deliver therapeutic mRNA to joint tissues, including cartilage, and to immune cells, supporting local therapeutic protein expression for musculoskeletal diseases such as osteoarthritis. We are also applying these delivery capabilities to advance cancer immunotherapy.
As below, the video below demonstrated green fluorescence labeled JBNp delivered into a cartilage cell.
4) Janus base nano-matrix (JBNm): tissue engineering and regenerative medicine on Earth and in space.
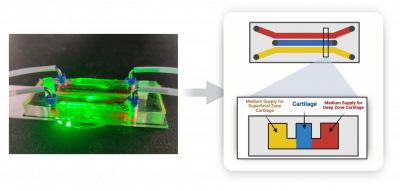
JBNm is an injectable scaffold formed by controlled assembly between JBNts, extracellular (ECM) proteins and growth factors. We can customize tissue-specific JBNms for different applications. For example, we have fabricated a cartilage-JBNm using matrilins (a cartilage ECM protein) and TGFβ (a cartilage growth factor). Importantly, JBNm is an injectable scaffold suitable for “difficult-to-reach” locations such as growth plate fractures in the center of a long bone and microchannels of microfluidic cartilage-on-a-chip.
Furthermore, we have pushed the boundary of bioengineering research bringing our technology from Earth into space. Supported by NASA, the International Space Station (ISS) national lab, NSF and other federal agencies, we develop and test our JBNs on the ISS via multiple space missions.
As below, the video demonstrated the self-assembly of JBNm in a biomimetic process without any chemical initiator or UV light.
5) In-Space Manufacturing of JBNs for Improved Outcomes on Earth
In partnership with Axiom Space, The ISS National Laboratory, and NASA, astronauts fabricate our JBNs aboard the International Space Station to help us investigate how we can utilize the unique conditions of microgravity in spaceflight to improve JBN assembly and their therapeutic outcomes in diseases of the cartilage (like osteoarthritis and growth plate injuries) and of the solid tumor (like breast and ovarian cancers). We have successfuly launched our materials on several space missions including AX-2, NG20, SpX30, SpX31, and SpX32 in just 2 years, with more missions already planned in the future!


